Properties and Applications of Ammonium Styrene Sulfonate (AmSS)
1.Introduction
SPINOMAR®NaSS (sodium styrene sulfonate) is a functional monomer developed using our proprietary bromination technology. NaSS and its polymers are applied in a wide range of fields, such as reactive emulsifiers for emulsion polymerization (for paints, coatings, adhesives, etc.), fiber dyeing auxiliaries, scale inhibitors, antistatic agents, cleaning dispersants, and conductive polymers. The manufacturing process of NaSS is shown in Fig.1. In this process, styrene undergoes an HBr anti-addition reaction to produce β-bromoethylbenzene (β-BEB), with subsequent sulfonation and dehydrobromination (vinylation) steps leading to NaSS production.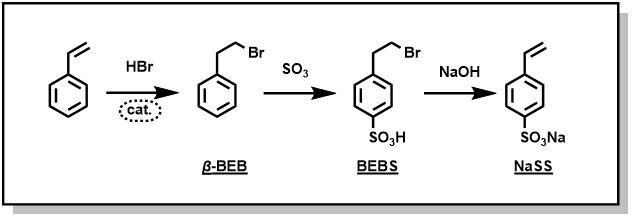
Fig.1 Manufacturing Process of NaSS
To diversify the functional properties of products, we have developed various types of styrene sulfonate salts (SS salts) beyond sodium salts. Among these, “Ammonium Styrene Sulfonate (AmSS)” has gained attention in recent years due to its unique structural characteristics (Table 1):
1. Metal-less composition(low metal content),
2. High solubility in various solvents.
AmSS is expected to find broader applications than NaSS, such as electronic materials and cation-exchange membranes. However, conventional production processes for AmSS faced challenges in industrialization due to quality and process limitations. Research on its fundamental properties and applications has been significantly limited.
In response to its high potential demand, we have recently developed an industrial-scale process for AmSS, alongside the active expansion of related business areas including NaSS operations. This paper provides an overview of the fundamental properties and applications of AmSS, which have rarely been reported previously, while incorporating a comparison with NaSS. The development of SS salts, including AmSS, aims to create functional monomers that can demonstrate versatility across different industries such as paints and conductive polymers. We are committed to using this development to build cross-functional technological foundations, contributing to industrial innovation and strengthening manufacturing infrastructure by addressing a wide range of material technology needs.
Table 1 Comparison of SS Salts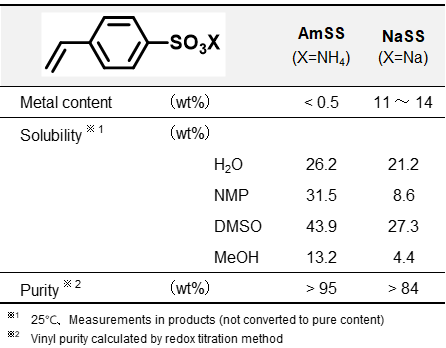
2.Properties
[1] General Properties
Table 2 General Properties and appearance of AmSS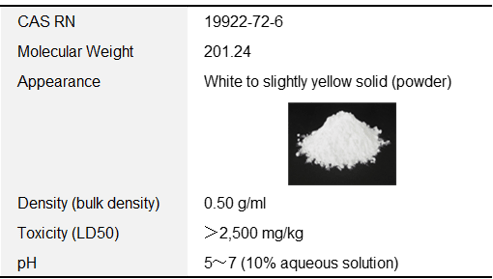
[2] Surface Activity
As mentioned in the introduction, NaSS is widely used as a reactive emulsifier in emulsion polymerization due to its high radical reactivity and moderate surface activity. Similarly, AmSS exhibits surfactant behavior comparable to NaSS (Fig.2), making it an equally suitable monomer for emulsion polymerization systems. Styrene sulfonate salts (SS salts), including AmSS, are well-suited for what are commonly referred to as soap-free (or more strictly, soap-less) emulsion polymerization systems, which utilize minimal amounts of conventional emulsifiers to enhance performance. Examples of applications in emulsion polymerization systems are further discussed in Section 3.[2].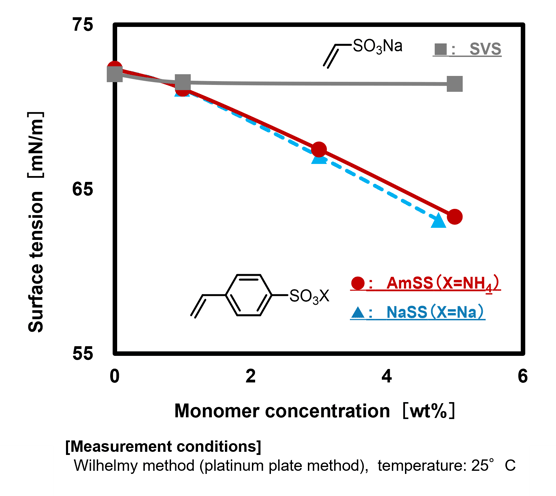
Fig.2 Surface tension of aqueous solution of sulfonated monomers
[3] Moisture Absorption (Hygroscopicity)
When comparing alkali metal salts with ammonium salts, it is generally observed that alkali metal salts exhibit higher hygroscopicity. This trend stems from the higher charge density and smaller ionic radius of alkali metal cations, which result in an increased number of hydration shells. Similarly, for styrene sulfonate (SS) salts, AmSS shows lower hygroscopicity compared to NaSS (Fig.3). The relationship between this property and its applications will be discussed in Section 4.[1]. 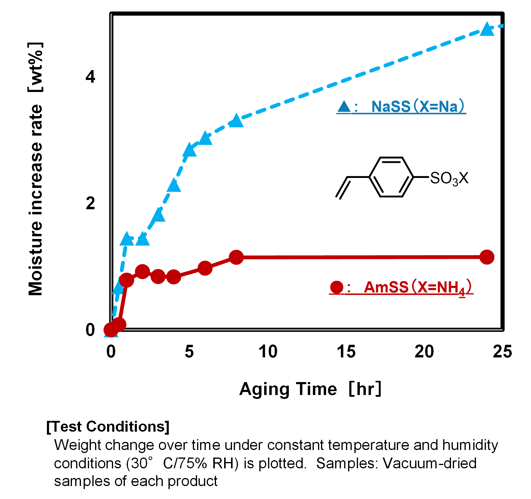
Fig.3 Hygroscopic Properties of SS Salts
[4] Thermal Stability
SS salts are known for their excellent heat resistance. AmSS specifically has a decomposition onset temperature of approximately 300℃, demonstrating its superior thermal stability.
[5] Storage Stability
Previously, AmSS was known for its low storage stability, which had been one of the primary quality-related issues identified (labeled as "Conventional Products" in Table 3). In this context, "storage stability" refers to the degree of deterioration in quality, such as heavyening, oxidation, discoloration, and other changes during long-term storage in its physical state.
Our research has revealed a negative correlation between the water content of AmSS and its storage stability, indicating that reduced water content leads to enhanced storage stability. Furthermore, other contributing parameters to storage stability were identified, allowing the development of specific compositions containing AmSS to achieve long-term storage stability (labeled as "Developed Products" in Table 3).
The stabilization mechanism involves multiple factors, making it difficult to explain definitively. One contributing factor is believed to be the nature of SS acid or its salts, which are prone to spontaneous polymerization under acidic conditions. Basic manufacturing methods for AmSS have already been discussed in previous publications and will not be detailed here; readers are encouraged to refer to publicly accessible documents as needed2.
Table 3 Storage Stability of AmSS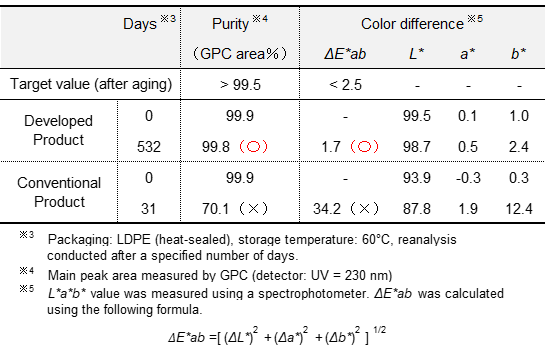
3.Polymerization
[1] Solution Polymerization
To investigate differences in polymerization characteristics in aqueous systems depending on cation type, polymerization reactions were conducted under identical conditions, except for the type of SS salt used.
(1) Polymerization Conditions
Two types of SS salt were used: NaSS and AmSS. The azo-based initiator 2,2'-azobis(2-methylpropionamidine) dihydro-chloride (V-50) was used as the polymerization initiator. To examine the impact of pH, additional systems involving AmSS supplemented with dimethylethanolamine (DMAE) or sodium hydroxide (NaOH) were also tested. A four-necked flask equipped with a condenser, thermometer, nitrogen inlet, and mechanical stirrer was used. SS salt (10.0 wt%), ion-exchanged water, and V-50 (0.15 mol%) were collectively introduced into the flask, degassed using nitrogen purging, and heated to 75°C. The conversion rate was measured periodically.
(2) Results
Comparison of the polymerization rates revealed that AmSS exhibits a faster polymerization rate compared to NaSS (Fig.4). The left vertical axis of Fig.4 represents the polymerization conversion rate, while the right vertical axis shows the polymerization rate calculated from monomer consumption. The parameter "k" denotes the polymerization rate coefficient.
Although ionic monomers typically show polymerization behavior strongly dependent on pH due to changes in polymer chain-end activity and monomer ionization, this result deviates from this general trend. As demonstrated in Fig.4, the polymerization rate of AmSS remains almost consistent regardless of pH. This behavior, which contradicts the widely observed pH-dependent ionic monomer polymerization tendency, is yet to be fully understood. Generally, pH sensitivity in ionic monomer polymerization is attributed to both the electrostatic repulsion between the polymer growth end and the monomer, which makes it difficult for the monomer to approach the active end, and the change in the reactivity of the monomer itself.3. However, these phenomena do not align directly with the behavior observed for AmSS.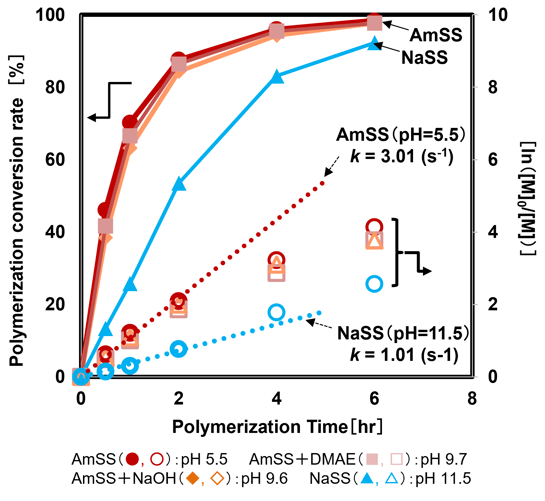
Fig.4 Polymerization Behavior of SS Salts
[2] Emulsion Polymerization
Soap-less emulsion polymerization of styrene was conducted using SS salts and emulsifiers under identical conditions, except for the type of SS salt. The polymerization conversion rate and emulsion quality (particle size distribution and degree of aggregation) were evaluated.
(1) Polymerization Conditions
Two types of SS salt were used: NaSS and AmSS. Ammonium persulfate (APS) served as the initiator, and ammonium dodecylbenzene sulfonate (DBS-NH4) was used as the emulsifier. A four-necked flask equipped with a condenser, thermometer, nitrogen inlet, and mechanical stirrer was utilized. Styrene (33.0 wt%), SS salt (1 mol%), APS (0.10 mol%), DBS-NH4 (0.02 mol%), and ion-exchanged water were introduced into the flask, degassed using nitrogen purging, and heated to 65°C. The conversion rate was measured periodically.
(2) Results
In styrene emulsion polymerization, both NaSS and AmSS functioned effectively as emulsifiers, producing high-quality emulsions. No significant differences were observed in polymerization conversion rates (Fig.5a) or particle size distributions (Fig.5b). Additionally, aggregation was minimal (below 0.2 wt%).
The results can be ascribed to the comparable surface activity and water solubility of NaSS and AmSS (Table 1, Fig.2). In the mechanism of emulsion polymerization, water-soluble monomers initially interact with free radicals generated by the water-soluble initiator. These radicals subsequently transfer into micelles stabilized by the emulsifier, enabling polymerization to proceed. SS salts play a dual role in this process: their water solubility supports monomer interactions with radicals in the aqueous phase, while their surface activity promotes interactions within the micelles, leading to comparable performance between NaSS and AmSS.
This result shows that SS salt structural units localize on particle surfaces, where sulfonic groups' electrostatic repulsion prevents aggregation and greatly enhances colloidal stability. Additionally, SS salt structural units are covalently grafted to particle surfaces, imparting superior resistance to physical stimuli (e.g., mechanical or shear stress), chemical stimuli (e.g., salt, organic solvents, and pH changes) and thermal stimuli (e.g., freezing) compared to conventional emulsifiers like DBS- NH44,5. As a result, using SS salts as emulsifiers enables the production of emulsions with superior stability.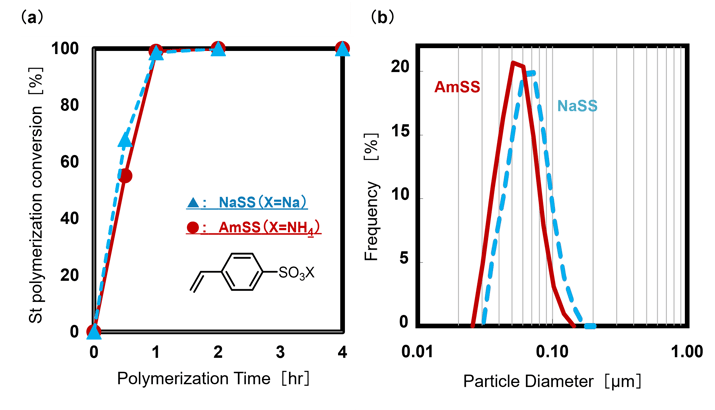
Fig.5 (a) Polymerization Conversion Rate of Styrene(St),
(b) Particle Size Distribution Measured via Dynamic Light Scattering
[3] Other Polymerization Systems
Although detailed descriptions are omitted in this paper, AmSS's solubility in various organic solvents allows solution polymerization with lipophilic monomers in organic media. AmSS excels in copolymerization with conjugated monomers like styrene and methacrylate.
Additionally, SS salts are also compatible with UV-induced photopolymerization and graft polymerization that utilize diverse activation sources6-8. In these polymerization systems, characteristics like dissolvability of additives (e.g., photosensitizers, initiators, plasticizers), substrate wettability, and activation species viability are crucial. The broader solvent compatibility of AmSS compared to other SS salts may often give it an advantage in these applications.
4.Applications
Previously, the limited availability of industrial-grade AmSS posed challenges to its widespread use, resulting in very few reported application examples. However, this lack of reporting is thought to stem not from insufficient potential utility but rather from challenges associated with production and procurement. With the advancements made through our research, AmSS is now expected to become more accessible, unlocking its promising applications across numerous fields. Therefore, this section aims to highlight several potential uses based on AmSS's unique material properties, categorized by characteristics.
[1] Metal-Free (Metal-less)
Polymerized forms of SS salts are expected to find applications in electronic materials, such as additives for chemical mechanical polishing (CMP) slurries or dispersants for carbon materials. In the field of electronic materials, metal constituents are generally undesirable because they can cause defects or corrosion. In this regard, the metal-free nature of AmSS makes it more favorable for such applications.
Additionally, beyond electronic materials, applications where NaSS has already been proven may benefit from advancements or problem-solving through the use of AmSS. One such example is waterborne coatings (emulsions) with improved water resistance. As described earlier, emulsions using NaSS as emulsifiers exhibit excellent colloidal stability, making them widely used as base resins for waterborne coatings. However, sodium-type emulsifiers sometimes face issues regarding water resistance, which is a critical factor affecting coating properties such as whitening, blistering, and mechanical strength.
Replacing NaSS with AmSS may offer a solution to these issues. Fig.6 presents the results of a water absorption test conducted on cast films of styrene (St)-butyl acrylate (BA)-SS salt random copolymers. In Fig.6, the vertical axis represents weight increase percentage, indicating higher water absorption (lower water resistance) with larger values and better water resistance with smaller values.
From the data shown in Fig.6, it is evident that increasing SS salt content leads to higher water absorption. Additionally, at the same SS salt content, ammonium salts exhibit lower water absorption compared to sodium salts. This corresponds to the hygroscopicity trends of SS salts discussed in Section 2.[3].
As reiterated in Section 3.[2], AmSS is equally applicable in emulsion polymerization as NaSS. Based on these findings, AmSS-based emulsions are expected to be an effective method for developing high-performance waterborne coatings with improved water resistance.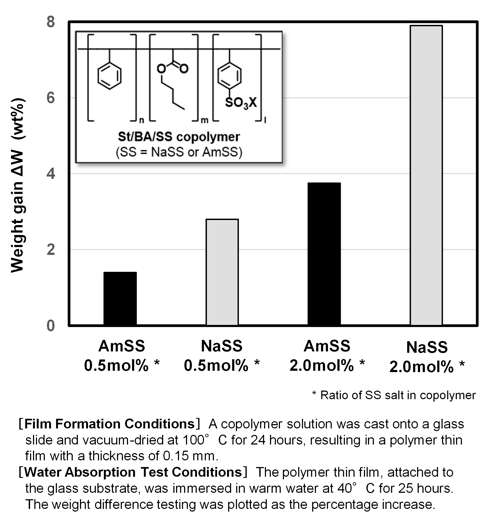
Fig.6 Comparison of Water Absorption Properties of SS Copolymers
[2] Solubility
AmSS, characterized by its solubility in organic solvents, is expected to facilitate improvements in the manufacturing processes of cation exchange membranes (ion exchange membranes). Among various types of cation exchange membranes, strong acidic hydrocarbon-based membranes often use styrene sulfonic acid frameworks as the exchange group. Conventionally, a post-sulfonation method is employed (Fig.7a)9,10, where styrene-based crosslinked materials are prepared first, followed by sulfonation using sulfonating agents to introduce sulfonic acid groups.
However, this post-sulfonation method faces several challenges, including the use of environmentally harmful strong oxidants and high complexity in reaction control. In contrast, an alternative approach has been reported (Fig.7b)9,11, where pre-sulfonated SS salts are used to manufacture cation exchange membranes without the need for a separate sulfonation step. In this process, SS salts require high solubility in organic solvents, particularly regarding compatibility with crosslinking agents like divinylbenzene. As shown in Table 1, AmSS exhibits higher solubility in highly polar solvents compared to NaSS, which suggests that it has the potential to sufficiently meet this requirement.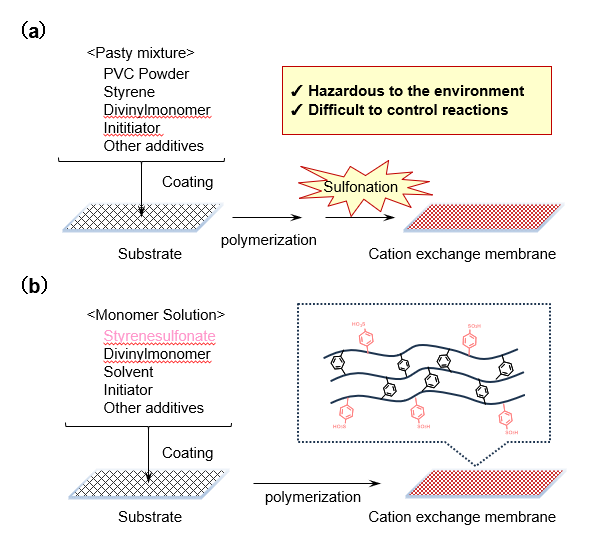
Fig.7 Illustration of Cation-Exchange Membrane Manufacturing Processes
(a) Post-Sulfonation Method9,10, (b) Improved Method Using SS Salts9,11
5.Summary
AmSS, while sharing similar physical properties with NaSS, demonstrates distinctive characteristics in terms of 1) alkali metal content, 2) solubility in organic solvents, and 3) radical polymerization reaction rate. Leveraging these features, AmSS has been increasingly applied in areas such as electronic materials, ion exchange membranes, and water-based coatings with improved water resistance. Its potential for expanded applications across various fields is anticipated in the future.
6.References
- 1) C.Salamone, Polymer Letters Edition,, 15, 487-491 (1971)
- 2) Tatsuo Hattori et al, Toyo Soda Kenkyu Hokoku, 24(1), 3-12 (1980)
- 3) Takao Hayashi et al, Toyo Soda Kenkyu Hokoku, 27(2), 81-86 (1983)
- 4) Sevilay Bilgin et al., European Polymer Journal, 93, 480-494 (2017)
- 5) Shinji Ozoe, Paint & Coatings Industry, 1-10 (2019)
- 6) Satoshi Tsuneda et al., Electrochem. Soc., 142(11), 3659-3663 (1995)
- 7) Shuhui Qin et al., Macromolecules, 37, 3965-3967 (2004)
- 8) Asmaa Attya Shalaby et al., Solid State Ionics, 404, 116420 (2024)
- 9) Stef Depuydt et al., Membranes, 14(1), 23 (2024)
- 10) Yukio Mizutani et al., Bulletin of the Chemical Society of Japan,
- 11) Ziliang Jia et al., Chemical Engineering Journal, 475(1), 146287 (2023)
- 12) Yusuke Shigeta et al, TOSOH Research & Technology Review, 47-52, 69(2025)
- SHARE

- Tweet
